What is a Battery?
I wanted to do an article on marine
batteries, but with me not being anywhere of an expert on the subject and after finding this
website, it appears to be so informative that I copied it here and am giving them
credit. In the past, I have just linked to good articles, only to later
find, that the link has died for what ever reason, never to be found again. The website
copied here is
http://www.windsun.com/Batteries/Battery_FAQ.htm
Look very carefully at the chart below.
The older 6 volt car batteries when they got low, would still turn the starter
over, but just a lot slower. But with the 12 volt, they need a near FULL charge,
with
anything below possibly 12.2 all you will get is maybe just the starter solenoid
clicking. Sure, it may run a radio but that takes a lot less power and is
not near that voltage sensitive.
|
State of
Charge
12 Volt
battery |
|
100% |
12.70 |
|
90% |
12.50 |
|
80% |
12.42 |
|
70% |
12.32 |
|
60% |
12.20 |
|
50% |
12.06 |
|
40% |
11.90 |
|
30% |
11.75 |
|
20% |
11.58 |
|
10% |
11.31 |
|
0 |
10.5 |
One thing to remember is
(probably twice a year) to remove the battery terminals from the battery,
clean them, check the wiring for any corrosion, clean and coat the connections
with a anti corrosion inhibitor. Even do this on your towing
vehicle so that you don't get in a situation where your vehicle may not start
at an inappropriate time.
----------------------------------------------------------------------------------------------------------------------------------------------------------------
A battery, in concept, can be any device that
stores energy for later use. A rock, pushed to the top of a hill, can be
considered a kind of battery, since the energy used to push it up the hill
(chemical energy, from muscles or combustion engines) is converted and stored as
potential kinetic energy at the top of the hill. Later, that energy is released
as kinetic and thermal energy when the rock rolls down the hill.
However not real
practical for everyday use though.
Common use of the word, "battery" in electrical
terms, is limited to an electrochemical device that converts chemical energy
into electricity, by a galvanic cell. A galvanic cell is a fairly simple device
consisting of two electrodes of different metals or metal compounds (an anode
and a cathode) and an electrolyte (usually acid, but some are alkaline)
solution. A "Battery" is two or more of those cells in series, although many
types of single cells are usually referred to as batteries - such as flashlight
batteries.
As noted above, a battery is an electrical
storage device. Batteries do not make electricity, they store it, just as a
water tank stores water for future use. As chemicals in the battery change,
electrical energy is stored or released. In rechargeable batteries this process
can be repeated many times. Batteries are not 100% efficient - some energy is
lost as heat and chemical reactions when charging and discharging. If you use
1000 watts from a battery, it might take 1050 or 1250 watts or more to fully
recharge it.
Internal Resistance
Part - or most - of the loss in charging and
discharging batteries is due to internal resistance. This is converted to heat,
which is why batteries get warm when being charged up. The lower the internal
resistance, the better.
Slower charging and discharging rates are more
efficient. A battery rated at 180 amp-hours over 6 hours might be rated at 220
AH at the 20-hour rate, and 260 AH at the 48-hour rate. Much of this loss of
efficiency is due to higher internal resistance at higher amperage rates -
internal resistance is not a constant - kind of like "the more you push, the
more it pushes back".
Typical efficiency in a lead-acid battery is
85-95%, in alkaline and NiCad battery it is about 65%. True deep cycle AGM's
(such as Concorde and Deka) can approach 98%.
Practically all batteries used in private vehicles and all but
the smallest backup systems are Lead-Acid type batteries. Even after over a
century of use, they still offer the best price to power ratio. A few systems
use NiCad, but we do not recommend them except in cases where extremely cold
temperatures (-50 F or less) are common. They are expensive to buy, and very
expensive to dispose of due the the hazardous nature of Cadmium.
We have had almost no direct experience with the
NiFe (alkaline) batteries, but from what we have learned from others we do not
not recommend them - one major disadvantage is that there is a large voltage
difference between the fully charged and discharged state. Another problem is
that they are very inefficient - you lose from 30-40% in heat just in charging
and discharging them. Many inverters and charge controls have a hard time with
them. It appears that the only current source for new cells seems to be from
Hungary.
An important fact is that ALL of the batteries
commonly used in deep cycle applications are Lead-Acid. This includes the
standard flooded (wet) batteries, gelled, and AGM. They all use the same
chemistry, although the actual construction of the plates etc varies.
NiCads, Nickel-Iron, and other types are found
in a few systems, but are not common due to their expense, environmental
hazards, and/or poor efficiency.
Major Battery Types
Batteries are divided in two ways, by
application (what they are used for) and construction (how they are built). The
major applications are automotive, marine, and deep-cycle. Deep-cycle includes
solar electric (PV), backup power, and RV and boat "house" batteries. The major
construction types are flooded (wet), gelled, and AGM (Absorbed Glass Mat). AGM
batteries are also sometimes called "starved electrolyte" or "dry", because the
fiberglass mat is only 95% saturated with Sulfuric acid and there is no excess
liquid.
Flooded may be standard, with removable caps, or
the so-called "maintenance free" (that means they are designed to die one week
after the warranty runs out). All gelled are sealed and are "valve regulated",
which means that a tiny valve keeps a slight positive pressure. Nearly all AGM
batteries are sealed valve regulated (commonly referred to as "VRLA" - Valve
Regulated Lead-Acid). Most valve regulated are under some pressure - 1 to 4 psi
at sea level.
Lifespan of Batteries
The lifespan of a deep cycle battery will vary
considerably with how it is used, how it is maintained and charged, temperature,
and other factors. In extreme cases, it can vary to extremes - we have seen
L-16's killed in less than a year by severe overcharging, and we have a large
set of surplus telephone batteries that sees only occasional (5-10 times per
year) heavy service that are now over 25 years old. We have seen gelled cells
destroyed in one day when overcharged with a large automotive charger. We have
seen golf cart batteries destroyed without ever being used in less than a year
because they were left sitting in a hot garage without being charged. Even the
so-called "dry charged" (where you add acid when you need them) have a shelf
life of 18 months at most. They are not totally dry - they are actually filled
with acid, the plates formed and charged, then the acid is dumped out.
These are some typical (minimum - maximum)
typical expectations for batteries if used in deep cycle service. There are so
many variables, such as depth of discharge, maintenance, temperature, how often
and how deep cycled, etc. that it is almost impossible to give a fixed number.
- Starting: 3-12 months
- Marine: 1-6 years
- Golf cart: 2-7 years
- AGM deep cycle: 4-7 years
- Gelled deep cycle: 2-5 years
- Deep cycle (L-16 type etc): 4-8 years
- Rolls-Surre tte premium deep cycle: 7-15 years
- Industrial deep cycle (Crown and Rolls 4KS series):
10-20+ years
- Telephone (float): 2-20 years. These are usually
special purpose "float service", but often appear on the surplus market as
"deep cycle". They can vary considerably, depending on age, usage, care, and
type.
- NiFe (alkaline): 5-35 years
- NiCad: 1-20 years
-
Starting, Marine, and
Deep-Cycle Batteries
- Starting
(sometimes called SLI, for starting, lighting, ignition) batteries are
commonly used to start and run engines. Engine starters need a very large
starting current for a very short time. Starting batteries have a large number
of thin plates for maximum surface area. The plates are composed of a Lead
"sponge", similar in appearance to a very fine foam sponge. This gives a very
large surface area, but if deep cycled, this sponge will quickly be consumed
and fall to the bottom of the cells. Automotive batteries will generally fail
after 30-150 deep cycles if deep cycled, while they may last for thousands of
cycles in normal starting use (2-5% discharge).
-
-
Deep cycle
batteries are designed to be discharged down as much as 80% time after time,
and have much thicker plates. The major difference between a true deep cycle
battery and others is that the plates are SOLID Lead plates - not sponge. This
gives less surface area, thus less "instant" power like starting batteries
need.
-
- Unfortunately, it is often impossible to tell what
you are really buying in some of the discount stores or places that specialize
in automotive batteries. The golf car battery is quite popular for small
systems and RV's. The problem is that "golf car" refers to a size of battery
(commonly called GC-2, or T-105), not the type or construction - so the
quality and construction of a golf car battery can vary considerably - ranging
from the cheap off brand with thin plates up the true deep cycle brands, such
as Crown, Deka, Trojan, etc. In general, you get what you pay for.
-
-
Marine
batteries are usually a "hybrid", and fall between the starting and deep-cycle
batteries, though a few (Rolls-Surrette and Concorde, for example) are true
deep cycle. In the hybrid, the plates may be composed of Lead sponge, but it
is coarser and heavier than that used in starting batteries. It is often hard
to tell what you are getting in a "marine" battery, but most are a hybrid.
Starting batteries are usually rated at "CCA", or cold cranking amps, or
"MCA", Marine cranking amps - the same as "CA". Any battery with the capacity
shown in CA or MCA may not be a true deep-cycle battery. It is sometimes hard
to tell, as the term deep cycle is often overused. CA and MCA ratings are at
32 degrees F, while CCA is at zero degree F. Unfortunately, the only positive
way to tell with some batteries is to buy one and cut it open - not much of an
option.
Using a Deep Cycle
battery as a Starting Battery
There is generally no problem with this,
providing that allowance is made for the lower
cranking amps
compared to a similar size starting battery. As a general rule, if you are going
to use a true deep cycle battery (such as the Concorde SunXtender) also as a
starting battery, it should be oversized about 20%
compared to the existing or recommended starting battery group size to get the
same cranking amps. That is about the same as replacing a group 24 with a group
31. With modern engines with fuel injection and electronic ignition, it
generally takes much less battery power to crank and start them, so raw cranking
amps is less important than it used to be. On the other hand, many cars, boats,
and RV's are more heavily loaded with power sucking "appliances", such as
megawatt stereo systems etc. that are more suited for deep cycle batteries. We
have used the Concorde SunXtender AGM batteries in some of our vehicles with no
problems.
It will not hurt a deep cycle battery to be used
as a starting battery, but for the same size battery they cannot supply as much
cranking amps as a regular starting battery.
Battery Construction
Materials
Nearly all large rechargeable batteries in
common use are Lead-Acid type. (There are some NiCads in use, but for most
purposes the very high initial expense, and the high expense of disposal, does
not justify them). The acid is typically 30% Sulfuric acid and 70% water at full
charge. NiFe (Nickel-Iron) batteries are also available - these have a very long
life, but rather poor efficiency (60-70%) and the voltages are different, making
it more difficult to match up with standard 12v/24/48v systems and inverters.
The biggest problem with NiFe batteries is that you may have to put in 100 watts
to get 70 watts of charge - they are much less efficient than Lead-Acid. What
you save on batteries you will have to make up for by buying a larger solar
panel system. NiCads are also inefficient - typically around 65% - and very
expensive. However, NiCads can be frozen without damage, so are sometimes used
in areas where the temperatures may fall below -50 degrees F. Most AGM batteries
will also survive freezing with no problems, even though the output when frozen
will be little or nothing.
Industrial Deep
Cycle
batteries
Sometimes called "fork lift", "traction" or
"stationary" batteries, are used where power is needed over a longer period of
time, and are designed to be "deep cycled", or discharged down as low as 20% of
full charge (80% DOD, or Depth of Discharge). These are often called traction
batteries because of their widespread use in forklifts, golf carts, and floor
sweepers (from which we get the "GC" and "FS" series of battery sizes). Deep
cycle batteries have much thicker plates than automotive batteries.
Plate Thickness
Plate thickness (of the Positive plate) matters
because of a factor called "positive grid corrosion". This ranks among the top 3
reasons for battery failure. The positive (+) plate is what gets eaten away
gradually over time, so eventually there is nothing left - it all falls to the
bottom as sediment. Thicker plates are directly related to longer life, so other
things being equal, the battery with the thickest plates will last the longest.
The negative plate in batteries expands somewhat during discharge, which is why
nearly all batteries have separators, such as glass mat or paper, that can be
compressed.
Automotive batteries typically have plates about
.040" (4/100") thick, while forklift batteries may have plates more than 1/4"
(.265" for example in larger Rolls-Surrette) thick - almost 7 times as thick as
auto batteries. The typical golf cart will have plates that are around .07 to
.11" thick. The Concorde AGM's are .115", The Rolls-Surrette L-16 type (CH460)
is .150", and the US Battery and Trojan L-16 types are .090". The Crown L-16HC
size has .22" thick plates. While plate thickness is not the only factor in how
many deep cycles a battery can take before it dies, it is the most important
one.
Most industrial deep-cycle batteries use Lead-Antimony plates rather than the
Lead-Calcium used in AGM or gelled deep-cycle batteries. The Antimony increases
plate life and strength, but increases gassing and water loss. This is why most
industrial batteries have to be checked often for water level if you do not have
Hydrocaps. The self discharge of batteries with
Lead-Antimony plates can be high - as much as 1% per day on an older battery. A
new AGM typically self-discharges at about 1-2% per month, while an old one may
be as much as 2% per week.
Sealed Batteries
Sealed batteries are made with vents that
(usually) cannot be removed. The so-called Maintenance Free batteries are also
sealed, but are not usually leak proof. Sealed batteries are not totally sealed,
as they must allow gas to vent during charging. If overcharged too many times,
some of these batteries can lose enough water that they will die before their
time. Most smaller deep cycle batteries (including AGM) use Lead-Calcium plates
for increased life, while most industrial and forklift batteries use
Lead-Antimony for greater plate strength to withstand shock and vibration.
A few industrial batteries have special caps
that convert the Hydrogen and Oxygen back into water, reducing water loss by up
to 95%. The popular "HydroCaps" that we sell for flooded batteries do the same
job for conventional ("wet"), golf cart, and fork-lift batteries. Lead-Antimony
(such as forklift and floor scrubber) batteries have a much higher
self-discharge rate (2-10% per week) than Lead or Lead-Calcium (1-5% per month),
but the Antimony improves the mechanical strength of the plates, which is an
important factor in electric vehicles. They are generally used where they are
under constant or very frequent charge/discharge cycles, such as fork lifts and
floor sweepers. The Antimony increases plate life at the expense of higher self
discharge. If left for long periods unused, these should be trickle charged to
avoid damage from sulfation - but this applies to ANY battery.
As in all things, there are trade offs. The
Lead-Antimony types have a very long lifespan, but higher self discharge rates.
Battery Size Codes
Batteries come in all different sizes. Many have
"group" sizes, which is based upon the physical size and terminal
placement. It is NOT a measure of battery capacity. Typical BCI codes are group
U1, 24, 27, and 31. Industrial batteries are usually designated by a part number
such as "FS" for floor sweeper, or "GC" for golf cart. Many batteries follow no
particular code, and are just manufacturers part numbers. Other standard size
codes are 4D & 8D, large industrial batteries, commonly used in solar electric
systems.
Some
common battery size codes used are: (ratings are approximate)
|
U1 |
|
34 to 40 Amp hours |
12 volts |
|
Group 24 |
10 1/4" OAL |
70-85 Amp hours |
12 volts |
|
Group 27 |
12 3/4" OAL |
85-105 Amp hours |
12 volts |
|
Group 31 |
13" OAL |
95-125 Amp hours |
12 volts |
|
4-D |
|
180-215 Amp hours |
12 volts |
|
8-D |
|
225-255 Amp hours |
12 volts |
|
Golf Cart & T-105 |
|
180 to 225 Amp hours |
6 volts |
|
L-16, L16HC etc. |
|
340 to 415 Amp hours |
6 volts |
Gelled electrolyte
Gelled batteries, or "Gel Cells" contain acid
that has been "gelled" by the addition of Silica Gel, turning the acid into a
solid mass that looks like gooey Jell-O. The advantage of these batteries is
that it is impossible to spill acid even if they are broken. However, there are
several disadvantages. One is that they must be charged at a slower rate (C/20)
to prevent excess gas from damaging the cells. They cannot be fast charged on a
conventional automotive charger or they may be permanently damaged. This is not
usually a problem with solar electric systems, but if an auxiliary generator or
inverter bulk charger is used, current must be limited
to the manufacturers specifications. Most better inverters commonly used in
solar electric systems can be set to limit charging current to the batteries.
Some other disadvantages of gel cells is that
they must be charged at a lower voltage (2/10th's less) than flooded or AGM
batteries. If overcharged, voids can develop in the gel which will never heal,
causing a loss in battery capacity. In hot climates, water loss can be enough
over 2-4 years to cause premature battery death. It is for this and other
reasons that we no longer sell any of the gelled cells except for replacement
use. The newer AGM (absorbed glass mat) batteries have all the advantages (and
then some) of gelled, with none of the disadvantages.
AGM, or Absorbed Glass Mat
Batteries
A newer type of sealed battery uses "Absorbed
Glass Mats", or AGM between the plates. This is a very fine fiber Boron-Silicate
glass mat. These type of batteries have all the advantages of gelled, but can
take much more abuse. We sell the Concorde (and Lifeline, made by Concorde) AGM
batteries. These are also called "starved electrolyte", as the mat is about 95%
saturated rather than fully soaked. That also means that they will not leak acid
even if broken.
AGM batteries have several advantages
over both gelled and flooded, at about the same cost as gelled:
Since all the electrolyte (acid) is contained in
the glass mats, they cannot spill, even if broken. This also means that since
they are non-hazardous, the shipping costs are lower. In addition, since there
is no liquid to freeze and expand, they are practically immune from freezing
damage.
Nearly all AGM batteries are "recombinant" -
what that means is that the Oxygen and Hydrogen recombine INSIDE the battery. These use gas phase transfer of oxygen to the negative plates to recombine them
back into water while charging and prevent the loss of water through
electrolysis. The recombining is typically 99+% efficient, so almost no water is
lost.
The charging voltages are the same as for any
standard battery - no need for any special adjustments or problems with
incompatible chargers or charge controls. And, since the internal resistance is
extremely low, there is almost no heating of the battery even under heavy charge
and discharge currents. The Concorde (and most AGM) batteries have no charge or
discharge current limits.
AGM's have a very low self-discharge - from 1%
to 3% per month is usual. This means that they can sit in storage for much
longer periods without charging than standard batteries. The Concorde batteries
can be almost fully recharged (95% or better) even after 30 days of being
totally discharged.
AGM's do not have any liquid to spill, and even
under severe overcharge conditions hydrogen emission is far below the 4% max
specified for aircraft and enclosed spaces. The plates in AGM's are tightly
packed and rigidly mounted, and will withstand shock and vibration better than
any standard battery.
Even with all the advantages listed above, there is still a place for the
standard flooded deep cycle battery. AGM's will cost 2 to 3 times as much as
flooded batteries of the same capacity. In many installations, where the
batteries are set in an area where you don't have to worry about fumes or
leakage, a standard or industrial deep cycle is a better economic choice. AGM
batteries main advantages are no maintenance, completely sealed against fumes,
Hydrogen, or leakage, non-spilling even if they are broken, and can survive most
freezes. Not everyone needs these features.
Temperature Effects on
Batteries
Battery capacity (how many amp-hours it can
hold) is reduced as temperature goes down, and increased as temperature goes up.
This is why your car battery dies on a cold winter morning, even though it
worked fine the previous afternoon. If your batteries spend part of the year
shivering in the cold, the reduced capacity has to be taken into account when
sizing the system batteries. The standard rating for batteries is at room
temperature - 25 degrees C (about 77 F). At approximately -22 degrees F (-27 C),
battery AH capacity drops to 50%. At freezing, capacity is reduced by 20%.
Capacity is increased at higher temperatures - at 122 degrees F, battery
capacity would be about 12% higher.
 Battery
charging voltage also changes with temperature. It will
vary from about 2.74 volts per cell (16.4 volts) at -40 C to 2.3 volts per cell
(13.8 volts) at 50 C. This is why you should have temperature compensation on
your charger or charge control if your batteries are outside and/or subject to
wide temperature variations. Some charge controls have temperature compensation
built in (such as Morningstar) - this works fine if the controller is subject to
the same temperatures as the batteries. However, if your batteries are outside,
and the controller is inside, it does not work that well. Adding another
complication is that large battery banks make up a large thermal
mass.
Battery
charging voltage also changes with temperature. It will
vary from about 2.74 volts per cell (16.4 volts) at -40 C to 2.3 volts per cell
(13.8 volts) at 50 C. This is why you should have temperature compensation on
your charger or charge control if your batteries are outside and/or subject to
wide temperature variations. Some charge controls have temperature compensation
built in (such as Morningstar) - this works fine if the controller is subject to
the same temperatures as the batteries. However, if your batteries are outside,
and the controller is inside, it does not work that well. Adding another
complication is that large battery banks make up a large thermal
mass.
Thermal mass means that because they have so
much mass, they will change internal temperature much slower than the
surrounding air temperature. A large insulated battery bank may vary as little
as 10 degrees over 24 hours internally, even though the air temperature varies
from 20 to 70 degrees. For this reason, external (add-on) temperature sensors
should be attached to one of the POSITIVE plate terminals, and bundled up a
little with some type of insulation on the terminal. The sensor will then read
very close to the actual internal battery temperature.
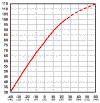 Even
though battery capacity at high temperatures is higher, battery life
is shortened. Battery capacity is reduced by 50% at -22 degrees F - but battery
LIFE increases by about 60%. Battery life is reduced at higher temperatures -
for every 15 degrees F over 77, battery life is cut in half. This holds true for
ANY type of Lead-Acid battery, whether sealed, gelled, AGM, industrial or
whatever. This is actually not as bad as it seems, as the battery will tend to
average out the good and bad times. Click on the small graph to see a full
size chart of temperature vs capacity.
Even
though battery capacity at high temperatures is higher, battery life
is shortened. Battery capacity is reduced by 50% at -22 degrees F - but battery
LIFE increases by about 60%. Battery life is reduced at higher temperatures -
for every 15 degrees F over 77, battery life is cut in half. This holds true for
ANY type of Lead-Acid battery, whether sealed, gelled, AGM, industrial or
whatever. This is actually not as bad as it seems, as the battery will tend to
average out the good and bad times. Click on the small graph to see a full
size chart of temperature vs capacity.
One last note on temperatures - in some places
that have extremely cold or hot conditions, batteries may be sold locally that
are NOT standard electrolyte (acid) strengths. The electrolyte may be stronger
(for cold) or weaker (for very hot) climates. In such cases, the specific
gravity and the voltages may vary from what we show.
Cycles vs Life
A battery "cycle" is one complete discharge and
recharge cycle. It is usually considered to be discharging from 100% to 20%, and
then back to 100%. However, there are often ratings for other depth of discharge
cycles, the most common ones are 10%, 20%, and 50%. You have to be careful when
looking at ratings that list how any cycles a battery is rated for unless it
also states how far down it is being discharged. For example, one of the widely
advertised telephone type (float service) batteries have been advertised as
having a 20-year life. If you look at the fine print, it has that rating only at
5% DOD - it is much less when used in an application where they are cycled
deeper on a regular basis. Those same batteries are rated at less than 5 years
if cycled to 50%. For example, most golf cart batteries are rated for about 550
cycles to 50% discharge - which equates to about 2 years.
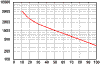 Battery
life is directly related to how deep the battery is
cycled each time. If a battery is discharged to 50% every day, it will last
about twice as long as if it is cycled to 80% DOD. If cycled only 10% DOD, it
will last about 5 times as long as one cycled to 50%. Obviously, there are some
practical limitations on this - you don't usually want to have a 5 ton pile of
batteries sitting there just to reduce the DOD. The most practical number to use
is 50% DOD on a regular basis. This does NOT mean you cannot go to 80% once in a
while. It's just that when designing a system when you have some idea of the
loads, you should figure on an average DOD of around 50%
for the best storage vs cost factor. Also, there is an upper limit - a battery
that is continually cycled 5% or less will usually not last as long as one
cycled down 10%. This happens because at very shallow cycles, the Lead Dioxide
tends to build up in clumps on the the positive plates rather in an even film.
The graph above shows how lifespan is affected by depth of discharge. The chart
is for a Concorde Lifeline battery, but all lead-acid batteries will be similar
in the shape of the curve, although the number of cycles will vary.
Battery
life is directly related to how deep the battery is
cycled each time. If a battery is discharged to 50% every day, it will last
about twice as long as if it is cycled to 80% DOD. If cycled only 10% DOD, it
will last about 5 times as long as one cycled to 50%. Obviously, there are some
practical limitations on this - you don't usually want to have a 5 ton pile of
batteries sitting there just to reduce the DOD. The most practical number to use
is 50% DOD on a regular basis. This does NOT mean you cannot go to 80% once in a
while. It's just that when designing a system when you have some idea of the
loads, you should figure on an average DOD of around 50%
for the best storage vs cost factor. Also, there is an upper limit - a battery
that is continually cycled 5% or less will usually not last as long as one
cycled down 10%. This happens because at very shallow cycles, the Lead Dioxide
tends to build up in clumps on the the positive plates rather in an even film.
The graph above shows how lifespan is affected by depth of discharge. The chart
is for a Concorde Lifeline battery, but all lead-acid batteries will be similar
in the shape of the curve, although the number of cycles will vary.
Battery Voltages
All Lead-Acid batteries supply about 2.14 volts
per cell (12.6 to 12.8 for a 12 volt battery) when fully charged. Batteries that
are stored for long periods will eventually lose all their charge. This
"leakage" or self discharge varies considerably with battery type, age, &
temperature. It can range from about 1% to 15% per month. Generally, new AGM
batteries have the lowest, and old industrial (Lead-Antimony plates) are the
highest. In systems that are continually connected to some type charging source,
whether it is solar, wind, or an AC powered charger this is seldom a problem.
However, one of the biggest killers of batteries is sitting stored in a partly
discharged state for a few months. A "float" charge should be maintained on the
batteries even if they are not used (or, especially if
they are not used). Even most "dry charged" batteries (those sold without
electrolyte so they can be shipped more easily, with acid added later) will
deteriorate over time. Max storage life on those is about 2-3 years.
Batteries self-discharge faster at higher
temperatures. Lifespan can also be seriously reduced at higher temperatures -
most manufacturers state this as a 50% loss in life for every 15 degrees F over
a 77 degree cell temperature. Lifespan is increased at the same rate if below 77
degrees, but capacity is reduced. This tends to even out in most systems - they
will spend part of their life at higher temperatures, and part at lower.
Myth:
The old myth about not storing batteries on concrete floors is just that - a
myth. This story has been around for 100 years, and originated back when battery
cases were made up of wood and asphalt. The acid would leak from them, and form
a slow-discharging circuit through the now acid-soaked and conductive floor.
State of Charge
State of charge, or conversely, the depth of
discharge (DOD) can be determined by measuring the voltage and/or the specific
gravity of the acid with a hydrometer. This will NOT tell you how good (capacity
in AH) the battery condition is - only a sustained
load test
can do that. Voltage on a fully charged battery will read 2.12 to 2.15 volts per
cell, or 12.7 volts for a 12 volt battery. At 50% the reading will be 2.03 VPC
(Volts Per Cell), and at 0% will be 1.75 VPC or less. Specific gravity will be
about 1.265 for a fully charged cell, and 1.13 or less for a totally discharged
cell. This can vary with battery types and brands somewhat - when you buy new
batteries you should charge them up and let them sit for a while, then take a
reference measurement. Many batteries are sealed, and hydrometer reading cannot
be taken, so you must rely on voltage. Hydrometer readings may not tell the
whole story, as it takes a while for the acid to get mixed up in wet cells. If
measured right after charging, you might see 1.27 at the top of the cell, even
though it is much less at the bottom. This does not apply to gelled or AGM
batteries.
"False" Capacity"
A battery can meet all the tests for being at
full charge, yet be much lower than it's original capacity. If plates are
damaged, sulfated, or partially gone from long use, the battery may give the
appearance of being fully charged, but in reality acts like
a battery of much smaller size. This same thing can occur in gelled cells if
they are overcharged and gaps or bubbles occur in the gel. What is left of the
plates may be fully functional, but with only 20% of the plates left...
Batteries usually go bad for other reasons before reaching this point, but it is
something to be aware of if your batteries seem to test OK but lack capacity and
go dead very quickly under load.
On the table below, you have to be careful that
you are not just measuring the surface charge. To properly check the voltages,
the battery should sit at rest for a few hours, or you should put a small load
on it, such as a small automotive bulb, for a few minutes. The voltages below
apply to ALL Lead-Acid batteries, except gelled. For gel cells, subtract .2
volts. Note that the voltages when actually charging
will be quite different, so do not use these numbers for a battery that is under
charge.
Amp-Hour Capacity
All deep cycle batteries are rated in amp-hours.
An amp-hour is one amp for one hour, or 10 amps for 1/10 of an hour and so
forth. It is amps x hours. If you have something that
pulls 20 amps, and you use it for 20 minutes, then the amp-hours used would be
20 (amps) x .333 (hours), or 6.67 AH. The accepted AH rating time period for
batteries used in solar electric and backup power systems (and for nearly all
deep cycle batteries) is the "20 hour rate". This means
that it is discharged down to 10.5 volts over a 20 hour period while the total
actual amp-hours it supplies is measured. Sometimes ratings at the 6 hour rate
and 100 hour rate are also given for comparison and for different applications.
The 6-hour rate is often used for industrial batteries, as that is a typical
daily duty cycle. Sometimes the 100 hour rate is given just to make the battery
look better than it really is, but it is also useful for figuring battery
capacity for long-term backup amp-hour requirements.
Why Amp-Hours are
Specified at a Particular Rate:
Because of something called the
Peukert Effect. The Peukert value is directly related to the internal
resistance of the battery. The higher the internal resistance, the higher the
losses while charging and discharging, especially at higher currents. This means
that the faster a battery is used (discharged), the LOWER the AH capacity.
Conversely, if it is drained slower, the AH capacity is higher. This is
important because some manufacturers and vendors have chosen to rate their
batteries at the 100 hour rate - which makes them look a lot better than they
really are. Here are some typical battery capacities from the manufacturers data
sheets:
|
Battery
Type |
100 hour
rate |
20 hour
rate |
8 |
|
Trojan T-105 |
250 AH |
225 AH |
n/a |
|
US Battery 2200 |
n/a |
225 AH |
181 AH |
|
Concorde PVX-6220 |
255 AH |
221 AH |
183 AH |
|
Surrette S-460 (L-16) |
429 AH |
344 AH |
282 AH |
|
Trojan L-16 |
400 AH |
360 AH |
n/a |
|
Surrette CS-25-PS |
974 AH |
779 AH |
639 AH |
State of Charge
Here are
no-load typical voltages vs state of charge
(figured at 10.5 volts = fully discharged, and
77 degrees F). Voltages are for a 12 volt battery system. For 24 volt systems
multiply by 2, for 48 volt system, multiply by 4. VPC is the volts per
individual cell - if you measure more than a .2 volt difference between each
cell, you need to equalize, or your batteries are going bad, or they may be
sulfated. These voltages are for batteries that have been at rest for 3 hours or
more. Batteries that are being charged will be higher - the voltages while under
charge will not tell you anything, you have to let the battery sit for a while.
For longest life, batteries should stay in the green zone. Occasional dips into
the yellow are not harmful, but continual discharges to those levels will
shorten battery life considerably. It is important to realize that voltage
measurements are only approximate. The best determination is to measure the
specific gravity, but in many batteries this is difficult or impossible. Note
the large voltage drop in the last 10%.+
|
State of
Charge |
12 Volt
battery |
Volts
per Cell |
|
100% |
12.7 |
2.12 |
|
90% |
12.5 |
2.08 |
|
80% |
12.42 |
2.07 |
|
70% |
12.32 |
2.05 |
|
60% |
12.20 |
2.03 |
|
50% |
12.06 |
2.01 |
|
40% |
11.90 |
1.98 |
|
30% |
11.75 |
1.96 |
|
20% |
11.58 |
1.93 |
|
10% |
11.31 |
1.89 |
|
0 |
10.50 |
1.75 |
Battery Charging
Battery charging takes
place in 3 basic stages: Bulk, Absorption, and
Float.
Bulk Charge
- The first stage of 3-stage battery charging. Current is sent
to batteries at the maximum safe rate they will accept until voltage rises to
near (80-90%) full charge level. Voltages at this stage typically range from
10.5 volts to 15 volts. There is no "correct" voltage for bulk charging, but
there may be limits on the maximum current that the battery and/or wiring can
take.
Absorption Charge:
The 2nd stage of 3-stage battery charging. Voltage remains constant
and current gradually tapers off as internal resistance increases during
charging. It is during this stage that the charger puts out maximum voltage.
Voltages at this stage are typically around 14.2 to 15.5 volts.
Float Charge:
The 3rd stage of 3-stage battery charging. After batteries reach full charge,
charging voltage is reduced to a lower level (typically 12.8 to 13.2) to reduce
gassing and prolong battery life. This is often referred to as a maintenance or
trickle charge, since it's main purpose is to keep an already charged battery
from discharging. PWM, or "pulse width modulation" accomplishes the same thing.
In PWM, the controller or charger senses tiny voltage drops in the battery and
sends very short charging cycles (pulses) to the battery. This may occur several
hundred times per minute. It is called "pulse width" because the width of the
pulses may vary from a few microseconds to several seconds. Note that for long
term float service, such as backup power systems that are seldom discharged, the
float voltage should be around 13.02 to 13.20 volts.
Chargers:
Most garage and consumer (automotive) type battery
chargers are bulk charge only, and have little (if any) voltage regulation. They
are fine for a quick boost to low batteries, but
not to leave on for long periods. Among the regulated chargers, there are the
voltage regulated ones, such as Iota Engineering and Todd, which keep a constant
regulated voltage on the batteries. If these are set to the correct voltages for
your batteries, they will keep the batteries charged without damage. These are
sometimes called "taper charge" - as if that is a selling point. What taper
charge really means is that as the battery gets charged up, the voltage goes up,
so the amps out of the charger goes down. They charge OK, but a charger rated at
20 amps may only be supplying 5 amps when the batteries are 80% charged. To get
around this, Statpower (and maybe others?) have come out with "smart", or
multi-stage chargers. These use a variable voltage to keep the charging amps
much more constant for faster charging.
Some of the newer chargers
will not charge DEAD battery, as they need about 3 volts to activate the
charger.
We carry the
Iota Engineering battery chargers and the
Statpower Truecharge "smart" chargers.
Charge Controllers
A charge controller is a regulator that goes
between the solar panels and the batteries. Regulators for solar systems are
designed to keep the batteries charged at peak without overcharging. Meters for
Amps (from the panels) and battery Volts are optional with most types. Some of
the various brands and models that we use and recommend are listed below. Note
that a couple of them are listed as "power trackers" - for a full explanation of
this, see our page on "Why
120 watts does not equal 120 watts".
Most of the modern controllers have automatic or
manual equalization built in, and many have a LOAD output. There is no "best"
controller for all applications - some systems may need the bells and whistles
of the more expensive controls, others may not.
These are some of the charge controllers that we recommend, but almost any
modern controller will work fine. Exact model will depend on application and
system size and voltage.
Xantrex (All)
Morningstar (All)
Outback Power MX60 & 80
Blue Sky Energy (Solar Boost)
Apollo
Steca
Using any of these will almost always give
better battery life and charge than "on-off" or simple shunt type regulators.
Battery Charging
Voltages and Currents:
Most flooded batteries should be charged at no
more than the "C/8" rate for any sustained period. "C/8" is the battery capacity
at the 20-hour rate divided by 8. For a 220 AH battery, this would equal 26
Amps. Gelled cells should be charged at no
more than the C/20 rate, or 5% of their amp-hour capacity. The Concorde AGM
batteries are a special case - the can be charged at up the the Cx4 rate, or
400% of the capacity for the bulk charge cycle. However, since very few battery
cables can take that much current, we don't recommend you try this at home. To
avoid cable overheating, you should stick to C/4 or less.
Charging at 15.5 volts will give you a 100%
charge on Lead-Acid batteries. Once the charging voltage reaches 2.583 volts per
cell, charging should stop or be reduced to a trickle charge. Note that flooded
batteries MUST bubble (gas) somewhat to insure a full charge, and to mix the
electrolyte. Float voltage for Lead-Acid batteries should be about 2.15 to 2.23
volts per cell, or about 12.9-13.4 volts for a 12 volt battery. At higher
temperatures (over 85 degrees F) this should be reduced to about 2.10 volts per
cell.
Never add acid to a battery except to replace
spilled liquid. Distilled or deionized water should be used to top off
non-sealed batteries. Float and charging voltages for gelled batteries are
usually about 2/10th volt less than for flooded to reduce water loss. Note that
many shunt-type charge controllers sold for solar systems will NOT give you a
full charge - check the specifications first. To get a full charge, you must
continue to apply a current after the battery voltage reaches the cutoff point
of most of these type of controllers. This is why we recommend the charge
controls and battery chargers listed in the sections above. Not all shunt type
controllers are 100% on or off, but most are.
Flooded battery life can be extended if an
equalizing charge is applied every 10 to 40 days. This is a charge that is about
10% higher than normal full charge voltage, and is applied for about 2 to 16
hours. This makes sure that all the cells are equally charged, and the gas
bubbles mix the electrolyte. If the liquid in standard wet cells is not mixed,
the electrolyte becomes "stratified". You can have very strong solution at the
top, and very weak at the bottom of the cell. With stratification, you can test
a battery with a hydrometer and get readings that are quite a ways off. If you
cannot equalize for some reason, you should let the battery sit for at least 24
hours and then use the hydrometer. AGM and gelled should be equalized 2-4
times a year at most - check the manufacturers recommendations, especially on
gelled.
Battery Aging
As batteries age, their maintenance requirements
change. This means longer charging time and/or higher finish rate (higher
amperage at the end of the charge). Usually older batteries need to be watered
more often. And, their capacity decreases.
Mini Factoids
Nearly all batteries will not reach full
capacity until cycled 10-30 times. A brand new battery will have a capacity of
about 5-10% less than the rated capacity.
Batteries should be watered after charging
unless the plates are exposed, then add just enough water to cover the plates.
After a full charge, the water level should be even in all cells and usually
1/4" to 1/2" below the bottom of the fill well in the cell (depends on battery
size and type).
In situations where multiple batteries are
connected in series, parallel or series/parallel, replacement batteries should
be the same size, type and manufacturer (if possible). Age and usage level
should be the same as the companion batteries. Do not put a new battery in a
pack which is more than 6 months old or has more than 75 cycles. Either replace
with all new or use a good used battery. For long life batteries, such as the
Surrette and Crown, you can have up to a one year age difference.
The vent caps on flooded batteries should remain
on the battery while charging. This prevents a lot of the water loss and
splashing that may occur when they are bubbling.
When you first buy a new set of flooded (wet)
batteries, you should fully charge and equalize them, and then take a hydrometer
reading for future reference. Since not all batteries have exactly the same acid
strength, this will give you a baseline for future readings.
When using a small solar panel to keep a float
(maintenance) charge on a battery (without using a charge controller), choose a
panel that will give a maximum output of about 1/300th to 1/1000th of the
amp-hour capacity. For a pair of golf cart batteries, that would be about a 1 to
5 watt panel - the smaller panel if you get 5 or more hours of sun per day, the
larger one for those long cloudy winter days in the Northeast.
Lead-Acid batteries do NOT have a memory, and
the rumor that they should be fully discharged to avoid this "memory" is totally
false and will lead to early battery failure.
Inactivity can be extremely harmful to a
battery. It is a VERY poor idea to buy new batteries and "save" them for later. Either buy them when you need them, or keep them on a continual trickle charge.
The best thing - if you buy them, use them.
Only clean water should be used for cleaning the
outside of batteries. Solvents or spray cleaners should not be used.
Observations By Writer
Not being an expert, but by simple and close observation, the "EverStart" batteries
from WalMart appear to be identical to the same size/model and ratings as Interstate batteries.
And the EverStart are considerably cheaper. As an example, the 27DC marine
battery at WalMart (3-2021) was $69.87, while the same size/model Interstate
battery at a well known national automotive parts store was $119.95.
|
Here a side by side comparison between EverStart &
Interstate batteries |
 |
More information -
Manufacturers Websites
US Battery Manufacturing Company - some good information
and data.
Crown Battery - A major manufacturer of industrial and deep
cycle batteries.
Trojan Battery - not a lot of real technical info here, but
has all the specifications.
Exide - not much here but marketing stuff, but you can buy
Exide T-shirts. We don't sell Exide.
Surrette - Specs and data on the Surrette deep cycle and
marine batteries
Concorde - specs and data on all the Concorde batteries,
including Lifeline.
Please feel free to
email us with any comments about this page or batteries in general.
Solar Electric Store
Discussion Forum
Solar Incentives
Home
Store
Links:
Solar Panels
Solar Panel Mounts
Solar Charge Controls
Deep Cycle Batteries
Battery Chargers
DC Lights
Wire & Cable
Fuses & Breakers
Wind Generators
Inverters
Solar Water Pumps
Hardware
Solar Internet Links
http://www.windsun.com/Batteries/Battery_FAQ.htm
********************************************************************************************************
For a look at marine battery usage concerning dual
batteries & or dual motors here is a link that has wiring diagrams of
different versions with the pros & cons of each. The section below was
taken from the following website message board
http://continuouswave.com/whaler/reference/dualBattery.html
Dual Battery Wiring
This article describes several techniques for interconnecting outboard motors
and dual batteries. There are two distinct applications: single engine
installations and dual engine installations.
Single Engine/Dual Battery
The use of a single engine and dual batteries is one of the most common
installations found in outboard powered boats. Interconnection is
straightforward. The diagram below shows the typical wiring.
 |
Dual Battery/Single Engine
Typical Schematic
NOTE: The battery positive leads are shown in red. The negative leads are
shown in green for clarity; code suggests the use of yellow wire. Wiring
gauge is determined by current demands and length of the run. Typically
2-AWG is used to connect to the batteries; 10-AWG is used to connect to the
distribution panel. The single switch is an OFF-1-BOTH-2 switch.
JWH. |
The use of a switch and wiring like this is recommend with dual battery
installations. A brief explanation of the operation of the switch in this
circuit follows.
In the OFF position, the two batteries are disconnected from all
loads. The OFF position is used when the boat is being stored or otherwise not
in use. It prevents any drain from the batteries. This can be useful if a
circuit has been accidently left on, say a cabin lighting circuit or similar
drain. Such a load can completely discharge a battery in a day or two, leading
to an unanticipated dead battery situation when you return to your boat. On some
switches a key lock is provided, allowing the switch to locked in the off
position. The provides another level of security in preventing the boat from
being used when left in storage or unattended.
In the 1 position, all loads and charging currents are connected to
the No. 1 battery (the PORT battery in the illustration). Starting current for
the engine starter motor is supplied by the PORT battery. When the engine is
running, surplus current developed by its charging circuit will flowing into the
PORT battery. Current to lighting and other loads will flow from the PORT
battery. The STDB battery is completely isolated and has no load current, nor
does it receive any charging current.
In the 2 position, all loads and charging currents are connected to
the No. 2 battery (the STBD battery in the illustration). Now it supplies
current required by starting and running, and it receives all the charging
current from the engine. The PORT battery is totally isolated.
In the BOTH position, the two batteries are connected in parallel.
This has a number of implications. Unless the batteries have exactly the same
state of charge, the combined voltage to the two batteries in parallel will sag
to a voltage somewhat lower than the highest battery's terminal voltage. Current
from the higher voltage battery will flow into the lower voltage battery and
begin charging it. As long as the state of charge in one battery is higher than
the other, the lower battery is more of a load than a source of power.
Eventually, the batteries will reach an equilibrium, and they will both have the
same terminal voltage. At that point they will both tend to supply current to
loads that are attached to them, and they will both receive charging current
furnished by the engine.
It would seem like operating in the BOTH position would be beneficial, but
that is not always the case. Even thought the batteries will eventually rise or
fall to the same terminal voltage when connected together, they will not
necessarily become exactly the same. A battery (or any source of electrical
engery) can be though of as having an internal resistance. The lower this
internal resistance the greater the current it can supply. The internal
resistance will also affect how the battery absorbs charging current. Even
though they are connected in parallel, it is possible that they will supply
unequal currents to the loads, and it is also possible that they will accept
unequal currents from an the engine charging source.
If the batteries are significantly different in their age, their type of
construction, and their state of charge, this unequal distribution of current
can be more significant. To describe the situation in the simplest of terms,
when two batteries are connected in parallel, they will probably tend to behave
more like the weakest battery of the two than the strongest.
Paralleling the batteries can come in handy in some situations. For example,
both batteries may be discharged to a point where neither alone can provide
enough current to crank the starter motor, but combined in parallel they can
turn the engine over.
If one battery is fully charged and the other is totally discharged,
connecting them in parallel (by using the BOTH position) can cause very high
currents to flow between the batteries. Extreme heat can be generated by the
sudden charging of the discharged battery. Use caution in this situation. It is
better to recondition a discharged battery by slowly re-charging it with an
AC-operated battery charger.
The arrangement of the contacts of the typical OFF-1-2-BOTH permits the
operation of the switch in the range of 1-2-BOTH without ever disconnecting the
batteries from the load or the outboard charging circuit. This is important, as
it is possible to cause damage to the charging circuit if the battery is
disconnected while the engine is running. By choosing the path of rotation of
the switch, it is possible to change from
1 to
2 without moving
through the OFF position.
Dual Engine/Dual Battery
Dual engines and dual batteries require careful interconnection to prevent
damage to the engine charging circuits. Three approaches are shown, one as
believed to be used by Boston Whaler, a second alternative approach (that I have
since discovered is described by West Marine in their catalogue), and a third
configuration which I am currently using in my Boston Whaler.
The Problem
The use of dual batteries in boats is quite common, but most often the dual
batteries are associated with just one engine or charging source. The wiring and
interconnection of two batteries and one engine alternator is quite well covered
in the boating literature. There is a less common and less written about
situation that arises when there are two batteries and two engines or charging
sources.
The problem with having two charging sources is that care must be taken to
not connect the two chargers together in a way that damages one or both of them.
Experience suggests that if two engines' chargers are wired in simple parallel
and charge a common battery or bank of batteries, it is not unusual for one of
the chargers to suffer a burned out stator coil. The cause of this is most
easily explained if a fundamental rule of charging circuits is understood: the
charger should never be operating without a load (a battery) connected.
In circuits with parallel connection of two engine charging circuits, it is
likely that the voltage produced by them will not be precisely equal. If one
unit has an output approximately one volt greater than the other, the effect of
this will be to electrically disconnect the load from the lower voltage output
engine. This may result in damage to the stator coil of the engine producing the
lower voltage output.
Boston Whaler Factory Installation
Several models of Boston Whaler boats come rigged from the factory with dual
outboards and dual engines. Below is the wiring which is believed to be
currently used by Boston Whaler in these installations.
 |
Dual Battery/Dual Engine
Schematic
NOTE: The starboard battery positive is shown in dark red for clarity; use
red wire. The negative leads are shown in green for clarity; use yellow
wire. All wiring is AWG-2 unless indicated. Switchs are OFF-1-2-BOTH style.
Adapted from a sketch supplied by Boston Whaler by JWH. |
Normal Operating Procedures
The Whaler Factory Installation operating procedure is believed to be:
- Normal operating position of PORT switch is Position
1
- Normal operating position of STBD Switch is Position
2
- To parallel batteries for starting, turn both switches to
ALL
Position. Return each switch to its normal operating position after starting.
-
- PORT engine can be started from STBD battery by turning PORT Switch to
Position 2. Return to Position
1 after starting.
- STBD engine can be started from PORT battery by turning STBD Switch to
Position 1. Return to Position
2 after starting.
If the suggested procedure is followed, the charging circuits of the two
engines will not be connected together in normal operation. One engine can
charge two batteries, but two engines should not charge a common battery.
Prolonged running of both engines operating on same battery is to be avoided;
prolonged running of both engines with both batteries paralleled is to be
avoided.
Alternate Dual Battery Configuration
An alternative approach that is not as flexible but is simpler to install and
operate is shown below.
 |
Dual Battery/Dual Engine
Alternative Schematic
NOTE: The starboard battery positive is shown in dark red for clarity; use
red wire. The negative leads are shown in green for clarity; use yellow
wire. All wiring is AWG-2 unless indicated. All switches are simple On-Off
switches.
JWH. |
Normal Operating Procedures
For the alternative dual battery wiring, the operating procedure is:
- For normal operation PORT switch is in
On position.
- For normal operation STBD switch is in
On position.
- For normal operation TIE switch is in
Off position.
- To parallel batteries for starting, turn TIE switch to
On position.
Return TIE switch to Off as soon as starting finished.
In some cases, the TIE Switch can be omitted and replaced by a temporary
connection between batteries using a jumper cable. It is probably a good idea to
carry jumper cables in any case. Connecting the two battery negative terminals
is also probably a good idea, even it the TIE switch is not used.
If the suggested procedure is followed, the charging circuits of the two
engines will not be connected together in normal operation. One engine can
charge two batteries, but two engines should not charge a common battery.
"New" Dual Battery Configuration
A new approach that I just implemented this spring on my Whaler is shown
below. Again, this arrangement is not as flexible as the "factory" wiring, but
it is the most simple and easiest to install.
 |
Dual Battery/Dual Engine
"New" Schematic
NOTE: The battery positive leads are shown in red. The negative leads are
shown in green for clarity; code suggests the use of yellow wire. All wiring
is AWG-2 unless indicated. The single switch is an OFF-1-BOTH-2 switch.
JWH. |
Normal Operating Procedures
In the "new" dual battery wiring, the two batteries
and the two engine
charging circuits are entirely isolated so long as the OFF-1-BOTH-2 switch is
NOT in the BOTH position. The switch serves a dual function. In the OFF position
it disconnects the house load from the batteries. In the 1 or 2 position, the
house load is powered from a single battery as selected. In the BOTH position,
the house load is powered from both batteries, and the two batteries are
connected in parallel. The BOTH position should only be used for special cases,
such as attempting to start an engine and needing additional battery power.
To prevent the paralleling of the engine charging circuits, when operating in
the BOTH position it is advisable to only run one engine at a time. The only
time the BOTH position may be needed is in starting an engine whose normal
battery is too weak to crank it over. In that case, the selector can be moved to
the BOTH position, temporarily paralleling the batteries and allowing the engine
(whose battery is weak) to be started.
Once the engine is running, the switch can be moved out of the BOTH position,
and the second engine started from its battery (which should have enough charge
remaining to crank it).
Thus the normal operating procedure is:
- For normal operation, select
OFF, 1, or 2 as appropriate to attach
the house load to a selected battery .
- To parallel batteries for starting, turn the switch to the
BOTH
position. Return the switch to OFF as soon as first engine starting
finished. Start second engine. If house load is needed connect to strongest
battery, either 1 or
2
-
If the suggested procedure is followed, the charging circuits of the two
engines will not be connected together in normal operation. One engine can
charge two batteries, but two engines should not charge a common battery.
Comments About the Three Designs
Whaler Factory
The more complex Whaler factory design allows for more flexibility, but at
the same time allows for the possibility that a wrong setting of the switches
could be made. The result could be damage to the engine charging circuitry. This
arrangement is probably most appropriate on larger, more complex vessels
Alternate
The principal advantage of the "alternate" wiring shown is its simplicity. If
the TIE switch is not thrown, there is no chance to connect two engine charging
circuits together. Sometimes in situations of high stress, like a hard starting
engine in a rough sea, simplicity has very desirable advantages.
Notice that the alternate arrangement connects the circuit breaker panel to
only the Starboard battery. This prevents the Port battery from being discharged
by other loads (like lamps left on or a stuck bilge pump switch), thus
preserving it for starting duty only. This can be an important advantage.
New
The principal advantage of the "new" wiring is its extreme simplicity. Only
one switch is used and it serves a dual purpose. The batteries and charging
systems are kept isolated except when the BOTH position is selected for
special or emergency starting conditions.
The disadvantage of this arrangement is there is no electrical disconnect of
the batteries from the engines. However, there are millions of outboard powered
boats that operate without such a disconnect, save for the case of opening the
battery box and removing the leads from the terminals. The only need for such a
disconnect would be in the unusual case of the engine electrical circuit
malfunctioning, as could occur with trim relay, starter motor contactor, or
other electrical load remaining stuck in an "ON" condition.
Other Wiring Arrangements
A great deal of other possible wiring and connection of batteries, loads,
switches, starters, chargers, and engines is possible. In many cases, additional
equipment like isolators and separate chargers are added. Some engine
alternators have specialized regulator circuits which permit separate sensing
leads to be connected to the battery to compensate for other voltage drops in
the circuit. In the main, none of this is applicable to outboard powered boats
for two very simple reasons. First, the starter motor and alternator are usually
connected together under the cowling of the outboard motor and are not easily
separated without significant electrical modification of the outboard. Second,
even if one were to re-wire their outboard motor, it would be necessary to add a
great deal of external wiring coming from the motor to the various external
components (like the isolators, the switches, the chargers, etc.). All this
wiring would have to be made flexible and be capable of withstanding the turning
and tilting inherent in the operation of an outboard motor.
In many years of boating, I have never heard of or seen anyone having
undertaken the modification of an outboard engine electrical under-cowling
wiring to interconnect isolators, switches, and other external circuit
components. If I did encounter such an outboard, I would be extremely wary of
it. I doubt that typical owners, do-it-yourself-er, or factory trained mechanics
would be comfortable working on such a modified engine. In short, I do not
endorse making that type of modification.
Caution Advised
Wiring of low-voltage/high-current circuits can be hazardous because of the
extremely high short circuit current capacity that exists when working with
large batteries. All the information provided is believed to be accurate and
representative of techniques and practices in common use in the marine
applications described. Examine any wiring carefully for proper configuration
before applying power. Low-voltage/high-current short circuits can produce
extremely rapid heating and can be dangerous. Never wear a ring or metal jewelry
when working with high-current sources like storage batteries.
Comments or Questions
If you have a comment or question about this article, please post it in the
Whaler Forum.
DISCLAIMER: This information is believed to be accurate but
there is no guarantee. We do our best!
The page has been accessed
63709 times.
Copyright © 1999, 2000 by James W. Hebert. Unauthorized reproduction
prohibited!
This is a verified HTML 4.0 document served to you from
continuousWave
URI: http://continuouswave.com/whaler/reference/dualBattery.html
Last modified: Saturday, 07-Feb-2009 23:08:01 EST
Author: James W. Hebert
This article first appeared February 10, 2001. A revised version appeared
February 14, 2001
Back to the Main
Ramblings Page
Updated 03-29-2021 ***
 Battery
charging voltage also changes with temperature. It will
vary from about 2.74 volts per cell (16.4 volts) at -40 C to 2.3 volts per cell
(13.8 volts) at 50 C. This is why you should have temperature compensation on
your charger or charge control if your batteries are outside and/or subject to
wide temperature variations. Some charge controls have temperature compensation
built in (such as Morningstar) - this works fine if the controller is subject to
the same temperatures as the batteries. However, if your batteries are outside,
and the controller is inside, it does not work that well. Adding another
complication is that large battery banks make up a large thermal
mass.
Battery
charging voltage also changes with temperature. It will
vary from about 2.74 volts per cell (16.4 volts) at -40 C to 2.3 volts per cell
(13.8 volts) at 50 C. This is why you should have temperature compensation on
your charger or charge control if your batteries are outside and/or subject to
wide temperature variations. Some charge controls have temperature compensation
built in (such as Morningstar) - this works fine if the controller is subject to
the same temperatures as the batteries. However, if your batteries are outside,
and the controller is inside, it does not work that well. Adding another
complication is that large battery banks make up a large thermal
mass.





